The O C H bond angle at C 1 should be approx 12 0 o and the terminal methyl group is in tetrahedral shape the bond angle within the methyl groups is 109. See the following examples for how to draw Lewis dot structures for common atoms involved in covalent bonding.
Write the correct skeletal structure for the molecule.

. The lewis structure of PF 5 contains a total of 5 bond pairs and 15 lone pairs3 lone pairs on each fluorine atom. Draw a skeleton and connect the atoms with single bonds. What kind of intermolecular forces act between a chloroform CHCl3 molecule and a chloroacetylene C2HCl molecule.
The Lewis Structure or Lewis Dot Diagram shows the bonding between atoms of a molecule and any electrons that may exist. In short these are the steps you need to follow for drawing a Lewis structure. 5 1 3 1 8.
Its reasonable to start off assuming the carbon atoms form only single bonds to. It is derived from acetic acid. Drawing Skeleton Structures.
The Lewis Structure for Li is Li with one dot to the right of the element. The drawing of the PF 5 lewiss structure is very easy and simple. Hydrogen atoms are always terminal only one bond Put more electronegative elements in terminal positions.
Those facts suggest the following general plan for drawing the Lewis structure of organic compounds. Who are the experts. It is used as a plasticizer hygroscopic agent and fire suppressant.
Draw the Lewis structure for chloroacetylene C2HCI. Draw the Lewis Dot Structure for the Hydrogen atom. The most difficult part of the four-step process in the previous section is writing the skeleton structure of the molecule.
View answer additonal benefits from the subscription. Learn more about Acetamide structure Properties Production and Uses with Frequently asked Questions here. Visit BYJUS for more content.
This type of Lewis dot structure is represented by an atomic symbol and a series of dots. Remember that H is never a. 100 13 ratings Transcribed image text.
Draw a skeleton using just the carbon atoms. Urine 68 and 60. Draw a skeleton using just the carbon atoms.
70 More Lewis Dot Structures. 1 3 2 4 1 3 14HCCH. Ć C - o X x s.
For example the molecule has two carbon atoms so you can begin by drawing this skeleton. Around iodine there are 7 valence electrons one of which is involved in the C I bond. Around each hydrogen there is 1 electron.
Ethanal is most likely a carcinogen in humans. As a general rule the less electronegative element is at the center of the molecule. Acetamide is the simplest amide.
The C H O group at the C 1 position is s p 2 hybridised and the terminal methyl groups are s p 3 hybridised. 1 1 2 4 1 1 10NH 3. The metabolism of inhaled 14 Cdichloroacetylene has been studied in male Wistar rats exposed to 20 or 40 ppm 78 or 156 mgcu m atmospheres for 1 hr.
Around each carbon there are 6 electrons 4 of which are involved in covalent bonds. Lewis Dot of Acetaldehyde ethanal CH 3 CHO. During the next 96 hr elimination of retained approximately 17 20 and 40 ppm doses respectively was.
The two Lewis structures suggest that one of the sulfur-oxygen bonds is stronger than the other. How to Draw the Lewis Dot Structure for C2H3Cl. Be certain you include any lone pairs.
Since all the atoms are in either period 1 or 2 this molecule will adhere to the octet rule. O C N and H- atoms form bond according to thier valency bulk atoms C N H valency - 2 - 3 bonds 2 4 3 so lewis structure - I O C N - C-H - two lone pair on H oxygen and one lone pair on nitrogen total 22 valence elections. Sum the valence electrons from all the atoms.
They follow the duet rule 2 electrons. Experts are tested by Chegg as specialists in their subject area. Since Hydrogen is in Group I it has one 1 valence electron in its shell.
A step-by-step explanation of how to draw the C2HCl Lewis Dot Structure ChloroethyneFor the C2HCl structure use the periodic table to find the total numbe. If there is more than one type of intermolecular force that acts be sure to list them all with a comma between the name of each force. Calculate the number of valence electrons.
PF 5 lewis structure is made up of five P-F bonds with a phosphorus P atom in a central position and all five fluorine F as outer atoms in the lewis diagram. We review their content and use your feedback to keep the quality high. Chloroethylene Vinyl chlorideFor the C2H3Cl structure use the periodic table to find the total number of va.
Chloroethane is the simplest and least toxic member of the class of chloroethanes that is ethane in which a single hydrogen is substituted by a chlorineA colourless gas at room temperature and pressure boiling point 12 it is used as a mild topical anaesthetic to numb the skin prior to ear piercing skin biopsies etc and is also used in the treatment of sports injuries. Each atom is thus neutral. 1 1 4 1 5 1 10H 3 CCH 3.
Feces 28 and 27. The exception of course being the hydrogens. Acetamide CH3CONH2- Acetamide is a chemical compound having the formula CH3CONH2.

How To Draw A Lewis Structure Lewis College Life Hacks Middle School Science

Ch4o Lewis Structure How To Draw The Lewis Structure For Ch4o Youtube

A Truncated Version Of The Periodic Table Showing Lewis Dot Structures For The First 20 Elements Hydr Chemistry Lessons Teaching Chemistry Chemistry Classroom
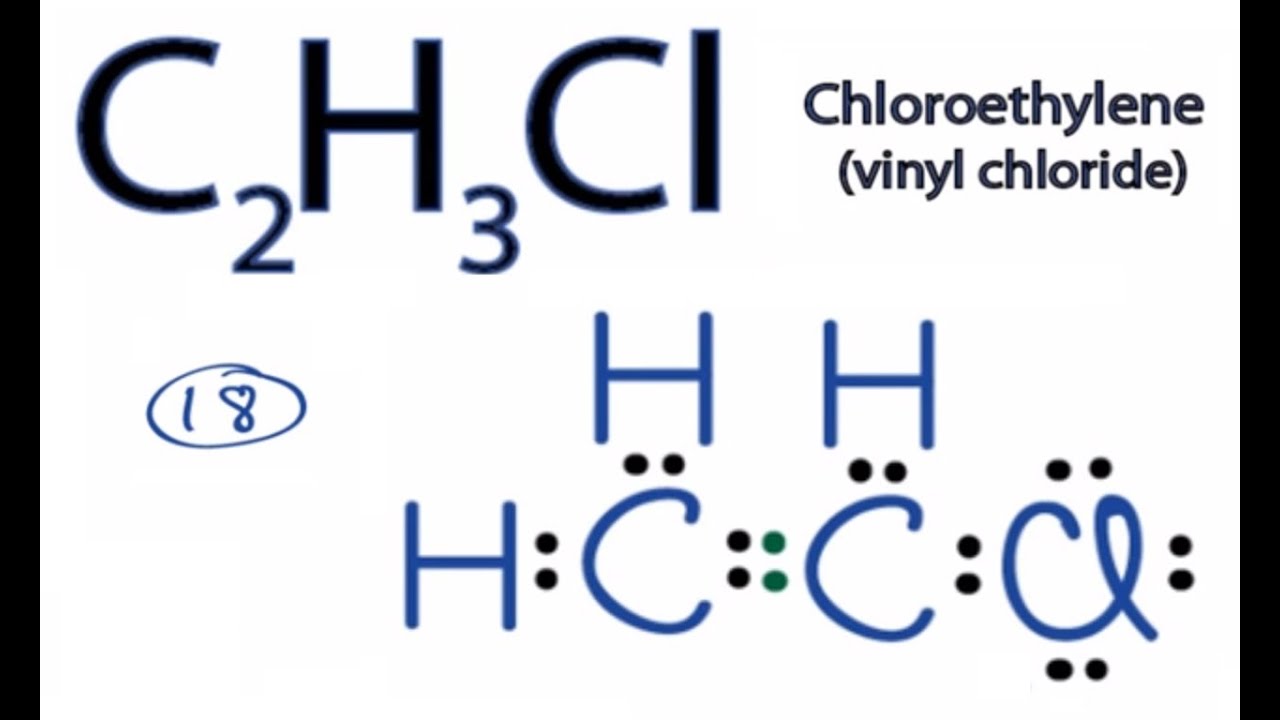
C2h3cl Lewis Structure How To Draw The Lewis Structure For C2h3cl Youtube

Lewis Structures Made Easy Examples And Tricks For Drawing Lewis Dot Diagrams Of Molecules Yo Teaching Science High School Chemistry Organic Chemistry Study

C2h3cl Lewis Structure How To Draw The Lewis Structure For C2h3cl Youtube

So4 2 Lewis Structure How To Draw The Lewis Structure For So4 2 Sulfate Ion This Step By Step Exp Chemistry Classroom Teaching Chemistry Chemistry Lessons
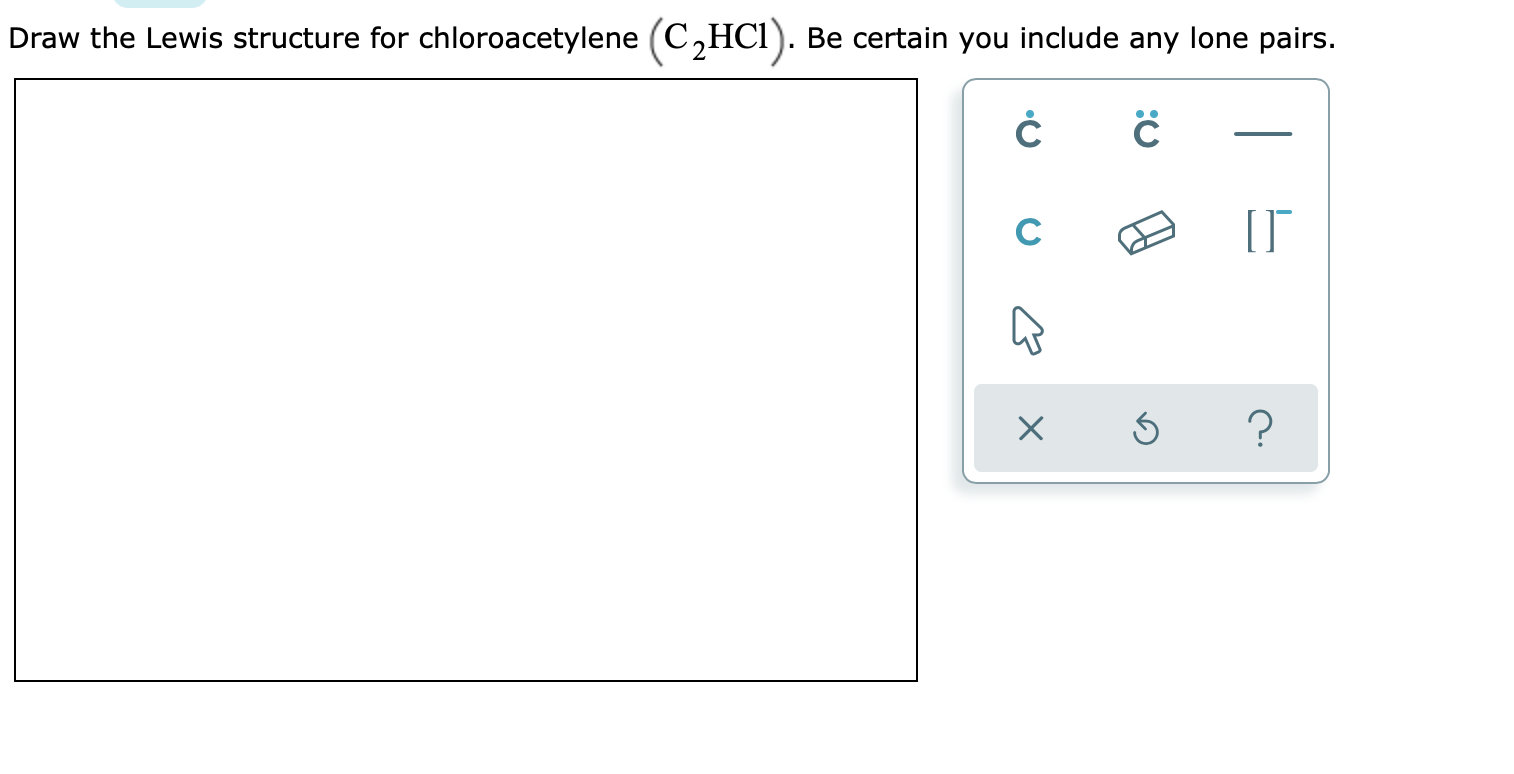
Solved Draw The Lewis Structure For Chloroacetylene C2hci Chegg Com

0 comments
Post a Comment